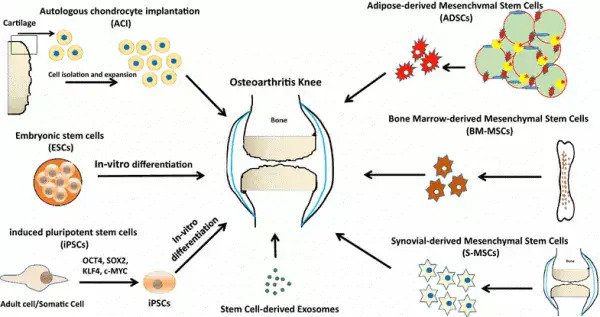
什么是骨关节炎?
骨关节炎(也称为退行性关节炎或退行性关节疾病)是一组与关节退化有关的机械性疾病。包括关节软骨和软骨下骨
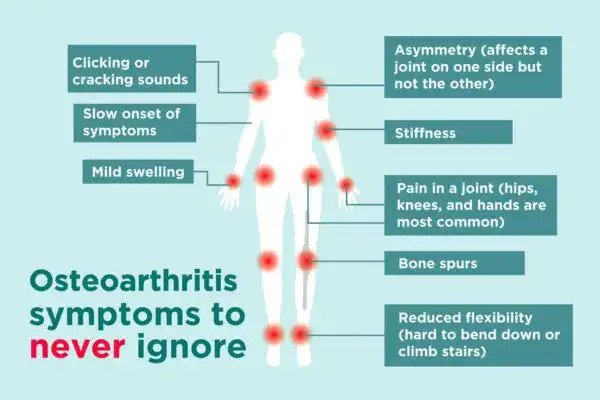
症状可能包括关节疼痛,压痛,僵硬,关节锁住,有时甚至是水浸。当骨骼表面没有正常雀斑的保护时。骨骼可能缺乏保护并变得受损。导致运动减少 某些区域的肌肉可能萎缩。韧带可能会变松
干细胞能治好骨关节炎吗?
干细胞疗法已被证明可诱导动物多种形式的关节炎的深入愈合过程,例如在某些使用干细胞加速基础疾病愈合的地方。
定期在马的诗句中 除了治愈受伤的组织 干细胞还具有调节免疫系统以阻止病理反应同时保持其抵抗疾病能力的特定能力。
哪些类型的干细胞用于治疗骨关节炎,如何收集它们?
用于治疗骨关节炎的成人干细胞来源于人的脐带组织。可以在正常健康的分娩后从供体脐带收集到。
每位母亲都要根据自己的病史进行筛查,并进行各种传染病检测。在捐赠之前,必须对所有脐带血干细胞进行最高标准的传染病筛查,以确保它们是真正安全的。